Seed to Sale Software – igwLAB
From receiving and extracting biomass to packaging a final product, a hemp processing business execution, quality management system and biomass tracking software must be streamlined. Enterprise Resource Planning software (ERP) is typically employed in a manufacturing setting to record and track hemp and CBD oil inventory.
extrakLAB saw the gaps related to integration and management of these manufacturing systems and created the igwLAB – a specialized tracking and manufacturing execution system (MES) designed for the cannabis industry. The seed to sale software is designed to aid managers, cost accountants and to work seamlessly with a company’s ERP systems with seamless integration to the Internet of Things (IOT).
What is the igwLAB Manufacturing Execution System?
igwLAB is a GMP-compliant, specialized quality tracking and seed to sale software built specifically for the hemp extraction industry. The igwLAB manufacturing execution system (MES) provides information to help manufacturing decision makers understand how current conditions on the plant floor can be optimized to improve production output.
igwLAB MES and seed to sale software was designed to support extraction facility operations manage material and information flow. The process of extracting hemp biomass into CBD or CBG oils requires extraction equipment, distillation equipment, and formulations operations to finish goods such as vapor pens or CBD pills.
The MES system includes thirteen unique individual modules that guides operators, quality managers, and laboratory personnel through the production process. The tracking software creates master batch records via quality analytics for each step in the process so that maximum traceability can be achieved and easily reported on.

Seed to Sale Software for Quality Management
There’s a lot of science intertwined with the extraction process. While you don’t need to be a chemist to execute, you do need a system that will help with tracking, and recording critical quality data.
Each piece of equipment in your extraction facility uses factors like temperature, pressure, time, volume and weight to shape an end product, manage risk, and comply with a rule or regulation.
Extraction isn’t just knowing what buttons to press on a piece of equipment. It’s about tracking all the data so you can manage your production and tell it where to go instead of wondering where it went. This is why management is crucial to your success as a manufacturer. As a busy entrepreneur, it is not possible to manage the complexity effectively with just a pen, a calculator, and a to-do list. This makes having a quality MES critical for information control in any business and in a variety of industries.
The Importance of GMP Compliance
The basic architecture of igwLAB is based on the foundation of FDA CFR Title 21 Part 2 that is responsible for governing food and drugs in the United States for the Food and Drug Administration and regulations set by the European Food Safety Authority (EFSA) and the European Medicines Agency (EMA).
This level of regulation compliance is incredibly important for producers who wish to sell their products to highly regulated markets like food, beverage and pharmaceuticals. Like all extraktLAB products, igwLAB is GMP compliant and built with components that support global market requirements.
How igwLAB Will Impact Your Extraction Facility Production
If you purchased a full solution from extraktLAB, you know that each piece of equipment is part of something larger, and that each piece of equipment in that process was specifically selected to increase efficiency, eliminate bottlenecks, and ensure quality management so that you don’t leave any money on the table.
igwLAB MES and hardware stations throughout your facility measure the performance of each of these stages individually and collectively through 34 different production reports utilizing nearly 100 data points. These reports give you the vital pulse of your extraction facility.
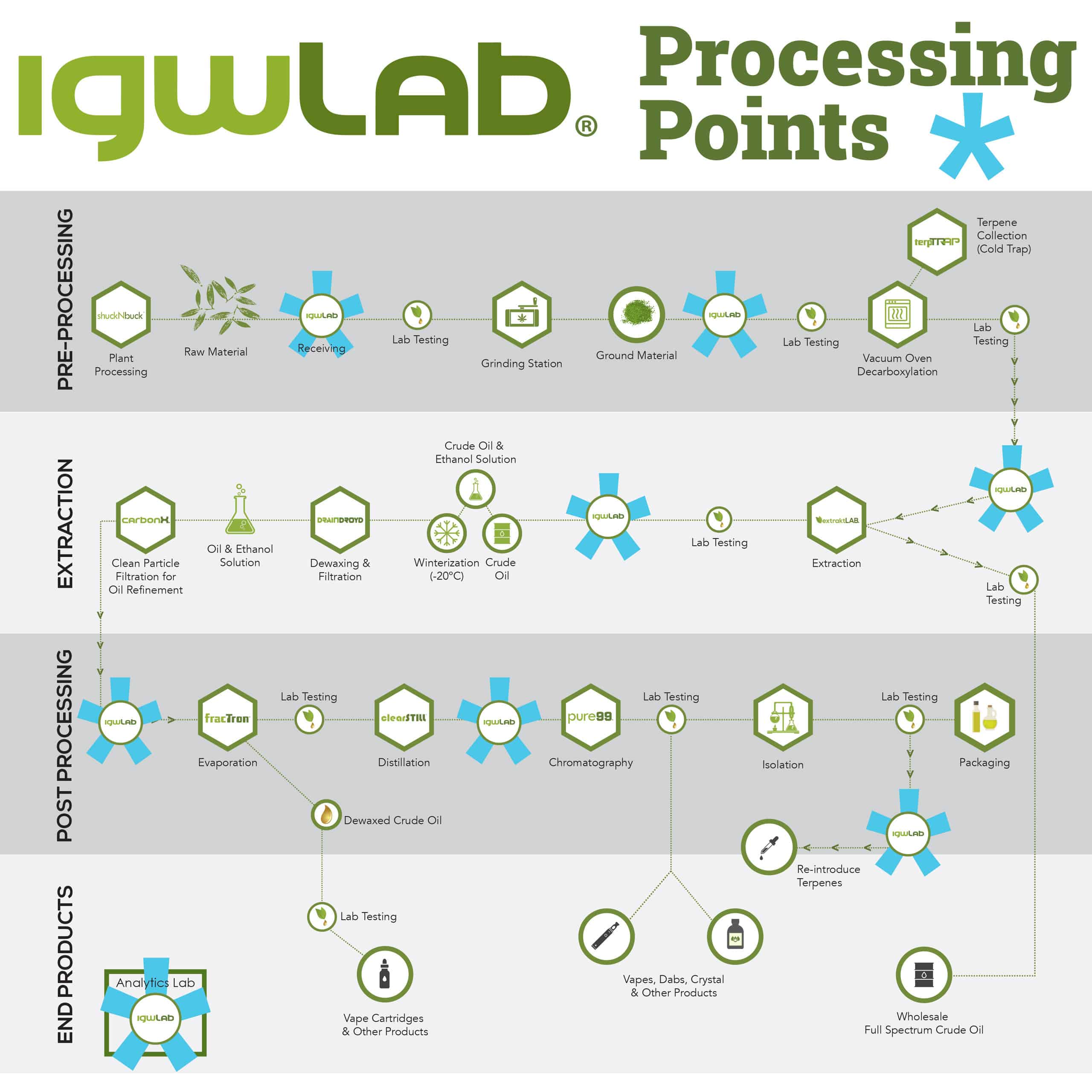
The production stages included with igwLAB MES are Receiving, Milling, Decarboxylation, Extraction, Dewaxing, Distillation, Formulation, Isolate, Filling and Packaging, Shipping, Analytical, Sampling. Each stage configuration was built specifically to fill the complex needs of a botanical extraction facility. You can expect a greater degree of measure and monitor control over each of the factors that impact these stages.

13 Hemp Process Control Modules
In order to monitor the production process step by step, igwLAB has 13 process control modules including:
- Bucking
- Milling
- Extraction
- Dewaxing
- Distillation
- Filing & Packaging
- Analytical
- Receiving
- Sampling
- Decarboxylation
- Isolate
- Formulation
- Shipping
Station Placement
igwLAB stations can be strategically placed throughout your facility to monitor your development production line. The recommended minimum number is 3, but depending on the complexity of your facility, you can add additional stations. See the igwLAB Processing Points example.
Adaptable Quality Management for Many Industries
As mentioned before, extraktLAB has taken the initiative to integrate key management systems with the hemp processing industry in mind. By doing so, igwLAB is built by hemp processors, for hemp processors. That being said, the tracking seed to sale software itself is incredibly adaptive and is capable of working with systems outside of extraktLAB’s production line and even outside the industry altogether.
Not only that, the system is applicable to many different industries. With only a slight modification, you could apply igwLAB to any type of manufacturing process. Take the food industry as an example. You would want to have raw inputs, making sure that the product is processed correctly at any certain point, that it’s being mixed to a certain degree, that it’s passing through ovens at a consistent temperature, etc.
For any manufacturer, igwLAB is capable of tracing the entire production line. This allows producers to access product line records from point A to point B and every step in between. Doing this ensures that productivity of any production process can be monitored, recorded and carefully analyzed for optimization and management of the entire process.
igwLAB Software Features & Process Control Modules
igwLAB FEATURES:
- GMP compliance Manufacturing Execution System with lot and batch control
- Cloud based seed to sale software platform with secure WIFI connectivity
- Yield optimization control
- Documentation and data management
- Customer and regulatory reporting
- Supports multiple locations
SOFTWARE FEATURES:
- Cloud Based Sales Software
- Validation Controls – 21 CFR Part 11
- Custom Integration with ERP/MRP with API
- Complete Lot Traceability & Tracking
HARDWARE FEATURES:
- IOT integration with scales, scanners, barcodes, printers, and readers.
- At bench consumable replenishing
- Plug & Play
13 PROCESS CONTROL MODULES:
- Bucking
- Milling
- Extraction
- Dewaxing
- Distillation
- Filing & Packaging
- Analytical
- Receiving
- Sampling
- Decarboxylation
- Isolate
- Formulation
- Shipping
igwLAB MES Software KEY MODULES
Manufacturing Execution
- Step-by-Step Instructions
- Mistake Proofing
- Value Added Time vs Non-Value Added Time
Quality Management
- Documentation Control
- SOPs & Documentation Delivery
- Documentation & Quality Record Generation
- Cleaning, Calibration, Maintenance, and Quality Events Records & Documentation
Laboratory Information
- Sampling Module
- Analysis Request & Barcoding
- Reporting Module & Links to Batches & Lots
Batch Records
- Automated Batch Records
- Integrated BOMs
- Packaging Lot & Automations
Testimonials
Fantastic service: speed, competence, thorough, and follow-up. UniSci/extraktLAB has helped me build my start-up company at a break neck pace, all while ensuring quality. Best kept secret in the industry.
Great team and great machines.
I just think you guys have the best technology hands down.
Good knowledge of the complete cycle, the individual components that make up the system, many questions answered, technical, commercial, financial.
Frequently Asked Questions
What does the software do?
- Reduces paperwork and human error
- Automates data collection on many routine tasks
- Automates quality records
- Manages production records and provides traceability
- Helps create logical workflow for each process.
- Provides the needed link between lab data and batch and process
- Document control
- Events manager
Why do I need this software?
- Pulls all the processes, procedures, quality systems, and material movements together in one software.
- Provides data dashboards to manage and adjust business.
- Provides a single place (infrastructure) at each step of the process to record process parameters.
What is the difference between igwLAB and METRC?
- METRC is a track and trace reporting tool for state systems
- igwLAB interfaces with METRC to via API
- igwLAB is different from Biotrack THC or MJ freeway as it does not have a retail or cultivation module.
How does IGW differ from other seed to sale software?
- Focuses specifically on manufacturing process
- Emphasis on batch records
- No POS attached to it
- No cultivation
What is the difference between igwLAB and process control software?
- Process control software records continuous physical parameters from manufacturing processes. The software continuously displays that data to show that the process is within pre-set statistical trends and confidence intervals. Process control algorithms built into the software use the data to adjust input independent variables to keep the process within said interval. Typically process control software is applied to complex multivariate processes or specific equipment.
- igwLAB records discontinuous physical, quality, lab data, SOPs, manufacturing actions, expiration dates, maintenance, events, locations, batch records, users, data and dashboards key process indicators.
What is the difference between igwLAB and LIMS or laboratory information management system?
- A laboratory information system manages samples, users, equipment, continuous data, laboratory quality parameters, results, and reports.
- Does not manage overall quality.
Is igwLAB an ERP?
- Not an ERP
- Feeds data into ERP
- No financial functions are included in the software
- No costing is included in software
- Can show inventory location, weights of waste, inputs, outputs and counts of final goods
What is included in the quality control module?
- Barcoded samples with location, batch, lot, location information along with sample data record.
- Sample testing request records.
- Sample testing results, reports, and automatic attachment to the batch or lot.
- Flag and alert system for out of specification or unexpected results.
What records does the system maintain?
Maintenance, calibration, cleaning, user, permissions, training, input weight, output weight, waste, vendors, biomass meta data, bill of materials, expiration dates, analytical results, sampling data, quality disposition, master batch record, standard operating procedures
What training is included?
- Online training
- Installation of hardware
- Online support ticket system
Is there a warranty?
All hardware included with igwLAB has warranty of 1 year from date of purchase.
How much work is it to implement?
The implementation process will take 30-60 days.
How does this work with my ERP system?
We have a well documented API
We can give you a quote to integrate your ERP and the igwLAB API or you are free to develop it on your own
How is hemp material transferred between areas to prevent contamination and material or batch mix up in hemp extraction?
Under GMP guidance, a risk assessment is conducted by management to identify those areas that are high risk for cross-contamination or batch mix up. Once the risk assessment is completed, process controls can be put in place to prevent contamination or Mix-Ups.
Sometimes the risk assessment might result in controls that can be as simple as a color coding or Designating a physical location for a particular material. More complex controls include in-process testing or double checks by quality assurance Durham Manufacturing.
Oftentimes the process controls and the process itself can be validated, with an approved validation protocol. Once the extraction method has been validated for mitigating the identified risk, cross-contamination and batch Mix-Ups typically only happen when process and procedure are ignored.
We use a software system called igwLAB to help us implement and mistake proof a validated manufacturing process. igwLAB is composed of both software and Hardware that works seamlessly together to give a full tracking solution where the movement of each lot or batch from one area is tracked with the software.
For example, a manufacturing process will not be able to process material without a release from quality assurance. Any attempts to do so will be flagged by the system and alert the operator to the mistake.
One thing that’s nice about the system is that it helps keep track of cleaning records and cleaning events for each piece of equipment that is put into the system. This can include tables, floors, ceilings, utensils, implements and Equipment. Any Surface that Can receive a barcode can be tracked in terms of cleaning events.
Standard operating Methods for cleaning these items are also stored in the software system so that the procedures can be readily accessed by the operator while the process is running. If the process calls for a cleaning event to be conducted, the software will not let the process proceed without cleaning for example.
Latest From The Blog
Third Party Certifications: How Safe is your CBD Product?
While it is always good practice to request third-party testing results for CBD products, they can’t always guarantee a pure and safe product. Denatured ethanol extraction can cause residual solvents and other materials to end up in the product at “acceptable” levels according to regulations. In this article we discuss what you need to know when it comes to third party testing and residual contamination in CBD products.
No Solute Left behind: Supercritical Fluids vs Liquid Solvents
In this article, extraktLAB R&D Chemical Engineer, Aaron Iwen, discusses the difference between supercritical fluids and liquid solvents and focuses on the primary factor for producers to consider when choosing their extraction solvents: solvation power. No...
What Parents Should Know About CBD
In this article, we will explore more about CBD and many of the positives and negatives associated with kids in the world of CBD, including present risks, unknowns and how parents can make the healthiest choices when considering CBD and their own children. Because the...
Get in touch with our team to request a quote, learn more about our training or get help with your business plan.
We are dedicated to providing you with the best advice, quality and service in the industry.



