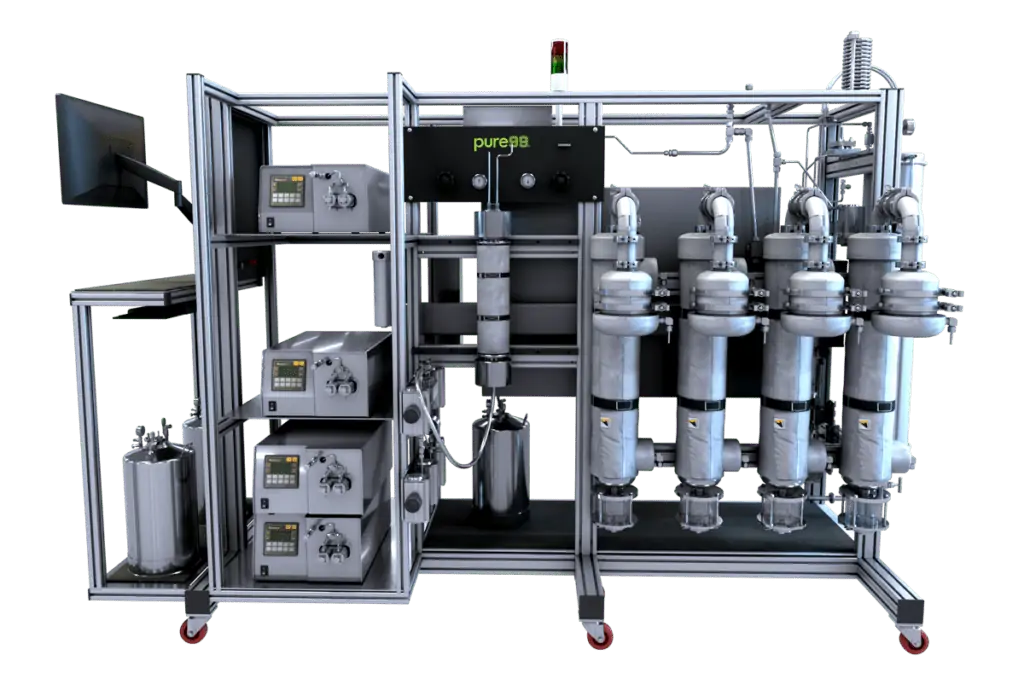
pure99-z: Column Chromatography System
Chromatography system designed for R&D and separations
The pure99-z system is an R&D platform that has a high degree of versatility. extraktLAB has developed this system as a highly efficient R&D tool that just keeps getting better and better. This highly versatile system enables multiple column packing an up to five fractions with high pressure injection and up to 100mL sample loop. It is the most efficient system for separations and research and development on the market.
Advanced Chromatography System For Separations and R&D
Features of the pure99-z
The pure99-z is the premier column chromatography system for separations, testing and R&D of hemp and cannabis extract products. There are several features that set the pure99-z apart from the competition:
- Ability to pack up to four columns
- Up to five different fractions
- High pressure injection with up to 100mL sample loop
- Pumps can pressurize up to 10,000 psi at 600-900 ml/min
- High pressure mixing eliminates solvent degassing and helium sparging
- 21 CFR Part 11 data integrity compliance.
- Selectivity for separation of cannabinoids.
- Stable stationary phases that are low-cost.
- State of the art data system enables methods automation and data integrity control
- GMP compliant and engineering standard compliant including C1D2 compliant, Atex II compliant, PED, ASME Section VIII DIV 2.

pure99 Column Upgrades
pure99 Stand Alone Columns provide separation flexibility that is required for every operation. The high maximum pressure and the variable column length allow optimization of the plate number and retention time. The unit comes as a complete assembly on casters that interfaces with the pure99 to control packed bed compression and provide eluent flow and sample injection.
Manufactured in the USA by United Science
| Dimensions |
Materials: | Inside Diameter
(cm) |
Max Pressure
(bar) |
Maximum Column Volume
(L) |
||
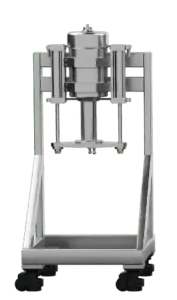
130 mm ID Column (1.5L) |
316 Stainless Steel, PTFE | 13 | 172 | 1.5 | ||
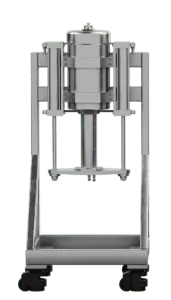
140 mm ID Column (3L) |
60.96 cm x60.96 cm x132 cm | 316 Stainless Steel, PTFE, Viton | 140 | 344 | 3 | Stand Alone Column on Casters |
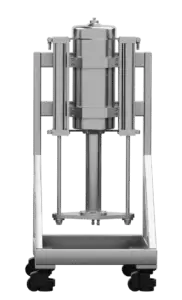
178 mm ID Column (7L) |
60.96 cm x60.96 cm x132 cm | 316 Stainless Steel, PTFE, Viton | 178 | 195 | 7 | Stand Alone Column on Casters |
178 mm ID Column (10L) |
60.96 cm x60.96 cm x132 cm | 316 Stainless Steel, PTFE, Viton | 178 | 195 | 10 | Stand Alone Column on Casters |
203 mm ID Column (13L) |
60.96 cm x60.96 cm x132 cm | 316 Stainless Steel, PTFE, Viton | 203 | 284 | 13 | Stand Alone Column on Casters |
Read Our Other Chromatography Publications
HPLC Applications
Online Product Tour
High-Performance Liquid Chromatography vs. Flash Chromatography
Water-free Separations
A primary concern associated with liquid-liquid extraction (CPC, CCC, LLE) is the use of both water and alcohol as one of the polar phases. The issue with this is that water is very tedious to remove from the end product being extracted. Because water has a very high boiling point while promoting the creation of an emulsified product, water should be avoided if at all possible. The methods used with the pure99-z system enables the paper used during the chromatography process to entirely avoid water during separation. This saves time, money, and mitigates the issues associated with water during CBD extraction.
Packable Columns
The chromatography columns that we sell can be packed by the customer in less than 10 minutes. The packing material itself is a low-cost compared other proprietary packing materials such as molecularly imprinted polymeric materials.
Unlike polymeric packing materials, our stationary phase is also mechanically stable so that the column can be run at high pressure.
Stationary phase is low cost and can be packed by the customer. The material is also highly mechanically stable so that the column won’t degrade or collapse at high pressure during the chromatography process.
High Yield
As with any chromatography method, there are always trade-offs between throughput, purity, and selectivity. Preparative chromatography yields a very high recovery rate compared other competing chromatography techniques. For example, it is not uncommon to recover up to 99% of the target cannabinoid. Liquid-liquid techniques typically have much lower recoveries. High recovery during chromatography depends primarily on the selectivity of separation.
But it can also depend on the peak shape and operational parameters such as where the chromatographer decides to make the cuts. Recovery largely depends on what is known in chromatography as selectivity. The greater the separation distance between cannabinoids, the greater the selectivity of the chromatography. The separation of cannabinoids through chromatography is never perfect. In other words, recovery can also depend upon how the chromatography operator selects the psychoactive cannabinoid.
High Production Output
The throughput of this chromatography system is 600-900 ml of extract produced per minute depending on the number of pumps. This flow rate depends on many factors including your column, your method, and your sample size. The great thing about the pure99-z is that production rate also includes the solvent recovery time for the eluent that is recoverable by the chromatography system.
Chromatography Methods, Training, SOPs Included
The pure99-z chromatography system is equipped with built in methods that are specifically tailored for the separation of cannabinoids. As a researcher, you will be able to determine the purity of your extract, the recovery that you wish to obtain and how many shifts you will have with the pure99-z. These selections and components will impact the overall throughput.
Equipment Specifications of the pure99-z
- 13.6 cm high pressure liquid separation chromatography column
- 20L collection vessels
- Chemical compatibility with organic solvents, acids and bases
- Pulse dampener and flow meter for precise flow control.
- High pressure pumping system up to 3000 psi.
- Full software control, automation, chromatography methods automation, and run reporting.
- Pre-pump eluent conditioner for high vapor pressure solvents.
- Packable media column.
| Attribute | Value |
| Number of Columns | Up to 4 |
| Flow Rate Range (LPM) | 600-900 ml/min |
| Maximum Allowable Working Pressure | 3000 psi |
| Eluent Inlet Lines | 2 or 3 |
| User Interface | Touch Screen 15″ |
| Automated Changeover and Sequences | Yes |
| Barcode Tracking | Yes |
| UV-Vis Detector Variable Wavelength | 210-600 nm |
Learn More About Our pure99-x Chromatography Machine
Frequently Asked Questions
Can I separate minor cannabinoids from major cannabinoids using the Pure99 Chromatography System?
We provide methods with the Pure99 system for THC and CBD separation. We also produce selective materials for CBN separation from THC/CBD. For other minor cannabinoids, the separation depends on how it will elute from the column in relation to the other major cannabinoids in the sample. That elution time and order depends on the column separation material known as the stationary phase, and the eluent, also known as the mobile phase. Scientists who are experts at finding unique conditions that will meet the goals of the separation are called chromatographers and they do methods development to find a suitable method.
What is the throughput of the Pure99?
Chromatography throughput for the specific example of the remediation of 1-3% THC in concentrated CBD can range from 1 kg to 6 kg per hour. Similar to distillation where the throughput will depend on the purity of the sample and how aggressive the cut is made, the throughput of a chromatography system is a trade off between purity of the final product and how aggressive the “cut” is made. It really depends on you. The general rule of thumb is that the sample will be more pure as the throughput decreases.
Do you provide SOPs for the Chromatography equipment?
Yes. We provide Standard operating procedures for analytical hplc, preparative hplc, GC headspace, and for sample preparation. We also provide start up kits for each one of these pieces of equipment along with sample management software called the igwLAB
Are the Chromatography Methods Validated for Cannabinoid Separation?
The customer validates the methods according to their company’s validation protocol. Chromatography and cleaning validation protocol for analytical methods are significantly different than production validation protocol and also very significantly from customer to customer.
Do Methods Come with the Pure99?
Yes. We provide the methods for separation of THC and CBD. If the customer desires to separate out other cannabinoids, they are welcome to develop their own methods.
What other Equipment is Required for the Pure99 to Function?
Other equipment and infrastructure besides the Pure99 is required to enable the proper functioning of a separations facility. These items include equipment that is either to measure how the method is doing overtime, equipment that is needed to measure how
HPLC with methods
fracTRON to remove solvents
Headspace GC with methods
Chillers and Heaters
Stainless or Glass Mixing Tanks
Drain Droid filtration
C1D2 Compliant Room
Solvent Storage Control Area
Is Cannabinoid Separation and Chromatography Training Included?
All of our chromatography products come with 2 days of training with optional on-site training at our demo facility in Osceola Wisconsin. Cannabinoid separation methods are also provided. Additional training for HPLC and for headspace GC is typically conducted at our facility in Osceola Wisconsin. On-site training for these items may be purchased by the customer.
Does distillation replace chromatography?
Chromatography and distillation are not analogous. Chromatography utilizes differences in chemical affinity to separate a mixture of components, distillation utilizes differences in boiling points. Wiped film distillation does not separate THC and CBD despite their differences in boiling points. Chromatography will separate THC and CBD into their pure fractions.
How do you remediate psychoactive cannabinoids from CBD oils?
There are several processes for the removal of psychoactive cannabinoids from CBD distillate. These include
1. Precipitation of CBD in a solvent
2. Chromatography separations.
3. Partitioning of cannabinoids in different organic solvents.
4. Degradation of psychoactive cannabinoids with oxidation and light.
In the isolate process, the CBD is precipitated from the oil in a nonpolar solvent blend Leaving the psychoactive cannabinoid in the supernatant to be discarded.
The second process is chromatography. In chromatography, cannabinoids are separated again in a solvent while flowing A packed bed of particles. This process can be frustrating for the end-user because it requires a high level of skill to conduct the separation. Oftentimes the particles can fail and plug up if the sample that is being separated has not been properly prepared. If done correctly, this process has the potential to separate out large amounts of CBD from psychoactive cannabinoids.
The third process involves separation by partitioning between two immiscible solvents. This process is very solvent intensive and is costly from the standpoint of solvent usage. There are also issues with solvent contamination of the final broad spectrum CBD oil. If done correctly, this process has the potential to separate out large amounts of CBD from psychoactive cannabinoids.
Finally, the last process involves degrading the Delta 9 into CBN with oxidation and uv light. The problem with this approach is that the CBD also degrades so the yield for this process is typically less than desirable.
Get in touch with our team to request a quote, learn more about our training or get help with your business plan.
We are dedicated to providing you with the best advice, quality and service in the industry.
Meet Our CEO and Founder Dr. Jon Thompson, Ph.D

Dr. Thompson is a separations scientist, entrepreneur, and inventor. As a scientist, he has a strong technical background and industry experience in analytical instrumentation, in-vitro diagnostics, biotech, mining, and homeland security markets. During his extraction industry career, Dr. Thompson has earned a strong track record of winning and implementing medical licenses in well-regulated, medically-modeled states. Dr. Thompson has assisted numerous companies to attain their goals in hemp manufacturing, as well as market development, strategic marketing, and worldwide business-to-business alliance formation (including international markets).