In this article, extraktLAB R&D Chemical Engineer, Aaron Iwen, discusses the difference between supercritical fluids and liquid solvents and focuses on the primary factor for producers to consider when choosing their extraction solvents: solvation power.
No extract is “solvent-less.” If an extract claims to be solvent-less, the separation of the “extract” from the starting material was achieved by physical means, not chemical. If removing target molecules from a biological matrix did not require chemical introduction, nobody would do it because it prompts additional downstream processing to remove the solvent which was intentionally introduced.
Therefore, everyone who gets into the hemp extraction business has to make a choice: Which solvent do I want to use? Many factors play into this decision: solvent consumption, capital cost, utility cost, labor requirements and extraction efficiency just to name a few. This article will focus on just one of these factors: solvation power.
So, What is Solvation Power?
Solvation power is a technical term for the ability of a solvent to solubilize a solute. This is a physical property unique to two individual compounds at a particular thermodynamic state. In this case, your solvent is whatever fluid you expose your biomass to, and your cannabinoids are your solute, which is extracted into your solvent. This is analogous to salt dissolving into water; water is your solvent, and salt is your solute.
In the scientific community, there is a phrase that is thrown around a lot which states “like dissolves like.” This is referring to your solvent and your solute. If your solute is similar enough to your solvent, it will dissolve it. While salt dissolves in water, oil does not. Therefore, solvation power varies depending on what your target solute is.
Solvation power can be manipulated by thermodynamics. Thermodynamics is the study of energy. When you think of energy, you might think of electricity, or heat. The most important energy to consider in an extraction process is chemical energy. Chemical energy is the force that dissolves your cannabinoids into your solvent. Chemical energy can be manipulated by changing temperature and pressure – the two fundamental variables in thermodynamics.
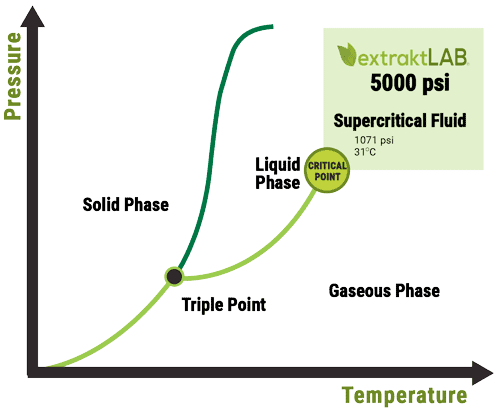
Is High Solvent Power a Good Thing?
Liquid solvents are regarded to have higher solvent power than supercritical fluids. Liquids have higher densities and it causes more solvent molecules to be exposed to a solute molecule. The solvent power of liquids is so high that it actually causes a disadvantage – more impurities are extracted. The over excessive solvent power of liquids requires that they operate at excessively low temperatures and greatly hinders the ability to manipulate the solvent. Solvent manipulation is important because, if it is done correctly, it allows for preferential extraction.
Supercritical fluids have the viscosity and density similar to that of a gas, but the fluidity of a liquid as shown in the above figure. This means that it permeates every last nook and cranny in your ground biomass to ensure that every microscopic cannabinoid solute is dissolved into the solvent. This characteristic is referred to as convective mass transfer and is largely forgotten in the discussion of solvent power.

In comparison, chilled liquid solvents have much higher viscosities which increases their surface tension, and decreases their convective mass transfer. This prevents liquid solvents from operating at the microscopic level effectively like supercritical fluids (see the figure above¹). At conditions common for liquid botanic extraction (i.e. ethanol at -70C), this can decrease your convective mass transfer ability by nearly 300% compared to room temperature conditions, and getting to those cold conditions doesn’t come without added utility cost². Having the ability to permeate even the most hard-to-reach places, supercritical fluids can extract biomass of any grind size without fear of leaving behind any cannabinoid solutes. Smaller grind size increases the amount of biomass that can be extracted in one run.
Benefits to Adding a Cosolvent
So far, the only factors mentioned consider a single solvent. The use of a cosolvent can chemically manipulate solvation power. By adding a cosolvent that has a different chemical structure, the solvent mixture has greater solvation power compared to a pure solvent. Supercritical systems commonly use a cosolvent to alter the solvation power and extract select compounds from their biomass.
Sometimes, adding a trace amount of liquid solvent can decrease extraction times, or allow for selectivity in your extraction depending on the thermodynamic conditions of the supercritical fluid. This allows you to maintain your high convective mass transfer, while manipulating your chemical compatibility for complete and precise extraction of your target molecules. Coupling a cosolvent’s ability to chemically manipulate solvation power with the heightened convective mass transfer capabilities of a supercritical fluid creates a solvent that can be manipulated beyond comparison.
Important Variables Affecting Solvation Power
Depending on your type of extraction solvent, there can be few or many variables that affect its solvation power. Liquids are primarily affected by temperature, of which decreasing negatively affects their convective mass transfer capabilities. Supercritical fluids are affected equally by co-solvent composition, temperature, and pressure.
Considering that individual manipulation of these three parameters could result in completely different values for solvation power, the amount of combinations for manipulating supercritical fluid solvents is multiples compared to liquid solvents. There is already confirmed literature³ of differences in CBD solubility in supercritical carbon dioxide at varying temperatures and pressures. With research on hemp being banned for decades, it’s only a matter of time.
– Aaron Iwen, MS Chemical Engineering
Want to know more about the benefits of supercritical CO2 extraction? Contact us or give us a call at 651-600-0036.
__________________________________________________________________________________________
REFERENCES:
1. “Production of solid lipid nanoparticles with a supercritical fluid assisted process.” The Journal of Supercritical Fluids. Accessed 23 Apr. 2020
2. “Ethanol – Online Services – DDBST.” Liquid Dynamic Viscosity Calculation by Vogel Equation (Ethanol). Accessed 23 Apr. 2020.
3. “Effects of Pressure in Supercritical CO2 Extraction of Cannabis.” Effects of Pressure in Supercritical CO2 Extraction of Cannabis. Accessed 23 Apr. 2020.
“Production of solid lipid nanoparticles with a supercritical fluid assisted process.” The Journal of Supercritical Fluids. Accessed 23 Apr. 2020


